Cerebral Palsy
Spastic cerebral palsy is the most common type of cerebral palsy, with 70% to 91% of motor disorders associated with cerebral palsy due to spasticity.1-5 In the U.S., nearly ¾ of a million children have cerebral palsy—50% (382,000) of these children suffer from severe spasticity.6
Unfortunately, the impact of spasticity is more devastating on children than adults because of the effects of muscle tightness on the growing musculoskeletal system. Muscle tightness can prevent normal bone and muscle development, resulting in substantial shortening of muscles, limb deformities, joint dislocations, and poor motor function, which occur when longitudinal muscle growth is restricted and tension is increased. Therefore, recognizing and implementing early treatment of spasticity in children with severe spasticity can potentially reduce the need for orthopedic surgery for contracture or torsion deformity.7

Three Kinds of Spastic Cerebral Palsy
Each type of spastic cerebral palsy should incorporate a different treatment plan to address the core disabilities and maximize functioning.1
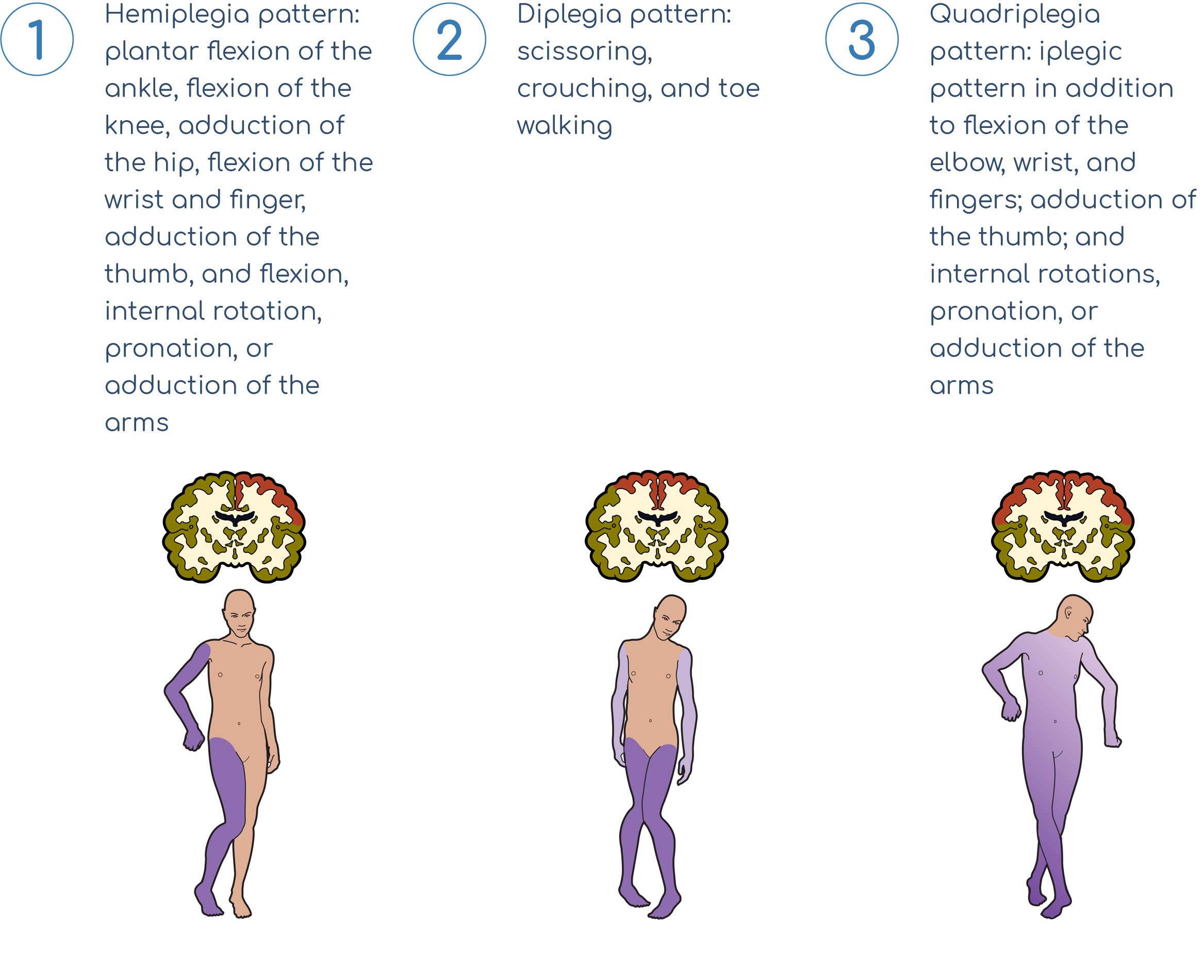
(Hemiplegia)
(Diplegia)
(Quadriplegia)
Spasticity Can Cause Major Functional Limitations
Equinovarus positioning of the foot is common in children with spastic cerebral palsy and causes major limitations in functional transfers or gait as the child grows.11
Supportive therapies, medications, and surgery may help to maximize a child’s functioning and alleviate discomfort.1 In children at least 4 years of age, consider implementing ITB Therapy℠ with Lioresal® Intrathecal (baclofen injection). Children who are being considered for ITB Therapy℠ with Lioresal® Intrathecal should be of sufficient body size to accommodate the implantable pump. The safety and efficacy of Lioresal® Intrathecal in children younger than 4 years has not been established.8
Choosing a therapeutic option like ITB Therapy℠ with Lioresal® Intrathecal has a well-established safety and efficacy profile and allows for a drug dosage and mode of delivery adjusted to the needs of each patient.2,8,9
Prior to implantation of a device for chronic intrathecal unfusion of Lioresal® Intrathecal Intrathecal (baclofen injection) patients must show a response to Lioresal® Intrathecal Intrathecal in a screening trial. It is mandatory that all patients, caregivers, and treating physicians receive adequate information regarding the risks of the mode of treatment. Instruction should be given on signs and symptoms of overdose, procedures to be followed in the event of an overdose, and proper home care of the pump and insertion site.
- Post-stroke rehabilitation. National Institute of Neurological Disorders and Stroke (NINDS) website. https://stroke.nih.gov/materials/rehabilitation.htm. September 2014. Accessed December 14, 2016.
- Hoving MA, van Raak EP, Spincemaille GH, et al. Efficacy of intrathecal baclofen therapy in children with intractable spastic cerebral palsy: a randomised controlled trial. Eur J Paediatr Neurol. 2009;13(3):240-246.
- Surman G, Bonellie S, Chalmers J, et al. UKCP: a collaborative network of cerebral palsy registers in the United Kingdom. J Public Health (Oxf). 2006;28(2):148-156.
- Reid SM, Carlin JB, Reddihough DS. Distribution of motor types in cerebral palsy: how do registry data compare? Dev Med Child Neurol. 2011;53(3):233-238.
- Hagberg B, Hagberg G, Beckung E, Uvebrant P. Changing panorama of cerebral palsy in Sweden. VIII. Prevalence and origin in the birth year period 1991-94. Acta Paediatr. 2001;90(3):271-277.
- McGuire JR. Chapter 2: Epidemiology of spasticity in the adult and child. In: Brashear A, Elovic E, eds. Spasticity: Diagnosis and Management. 2nd ed. New York, NY: Demos Medical Publishing, LLC, 2016.
- Saulino M, Ivanhoe CB, McGuire JR, et al. Best practices for intrathecal baclofen therapy: patient selection. Neuromodulation. 2016;19(6):607-615.
- Lioresal® Intrathecal (baclofen injection) [prescribing information]. Saol Therapeutics, Roswell, Georgia; January 2019.
- Kolaski K, Koman LA. Chapter 29: Surgical management of spasticity in the child with cerebral palsy. In: Brashear A, Elovic E, eds. Spasticity: Diagnosis and Management. 2nd ed. New York, NY: Demos Medical Publishing, LLC, 2016.
- Agarwal A, Verma I. Cerebral palsy in children: An overview. J Clin Orthop Trauma. 2012;3(2):77-81