Targeted Delivery for Effective Control
To be effective, baclofen must be present within the cerebral spinal fluid (CSF) to reach GABA B receptors on target neurons. However, baclofen does not readily cross the blood-brain barrier (BBB). Intrathecal baclofen (ITB) Therapy℠ with Lioresal® Intrathecal delivers baclofen directly to the CSF as opposed to systemic delivery with oral baclofen. This allows for rapid distribution of baclofen directly to target neurons, delivering symptom relief where spasticity arises.1,2
Because ITB therapy delivers baclofen directly to the site of action, it can provide more accurate and individualized dosing with lower baclofen dosages.1,2 With ITB Therapy℠ with Lioresal® Intrathecal (baclofen injection), there is 100 times less medication is in the blood compared to oral baclofen at recommended dosages.1 Specific concentrations of baclofen needed depend on the total daily dose required to treat severe spasticity. In clinical studies in patients with spinal cord injury, maintenance continuous infusion dosages have ranged from 12 to 2003 mcg/L, with most patients maintained on 300 to 800 mcg/L. Similarly, for patients with cerebral palsy, maintenance continuous infusion dosages have ranged from 22 to 1900 mcg/L, with most patients maintained on 90 to 703 mcg/L. In these patients, plasma concentrations are expected to be 0 to 5 mcg/L.1
Stable dosages can be maintained long-term. In a cross-sectional survey of patients receiving ITB therapy or oral baclofen, the mean change in ITB dosages ranged from 3% to 5% over 3 years, whereas the mean change in oral dosages ranged from 9% to 21%.3
Difference in Dosages in a 3-Year Study of ITB Therapy vs Oral Baclofen3
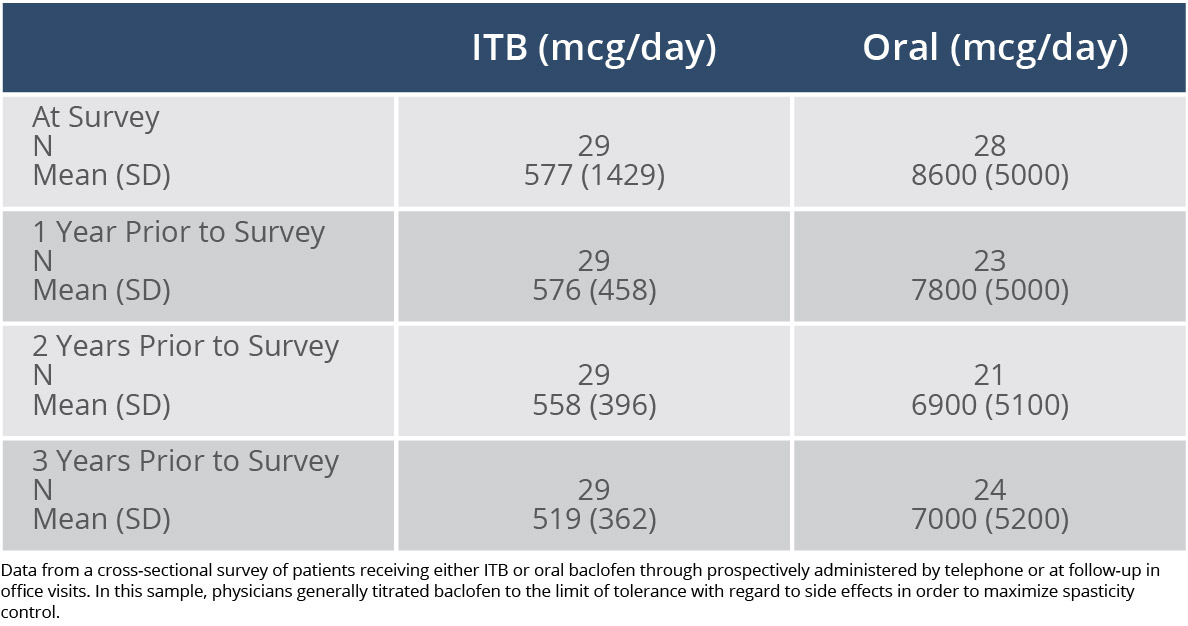
Adapted from Reference 3: McCormick ZL, Chu SK, Binler D, et al. Intrathecal versus oral baclofen: a matched cohort study of spasticity, pain, sleep, fatigue, and quality of life. PM R. 2016;8(6):553-562.
Importantly, even with a lower dose, spasticity is significantly less frequent and less severe with ITB therapy. With bolus dosing of baclofen, ITB therapy resulted in an improvement in Modified Ashworth Scale (MAS) scores within 1 hour, with maximal effect generally occurring 4 hours after dosing. This effect typically lasts 4 to 8 hours. With continuous infusion, antispastic activity is first seen at 6 to 8 hours after initiation and lasts 24 to 48 hours. Mean spasm frequency and severity scores with ITB therapy indicated spasms only occurred with stimulation and were mild in severity. In contrast, with oral baclofen, scores indicated infrequent but full spasms that occurred less than once per hour and were moderate in severity.1,3
MAS Scores After Continuous Dosing of Oral Baclofen and Bolus Dosing with ITB Therapy4
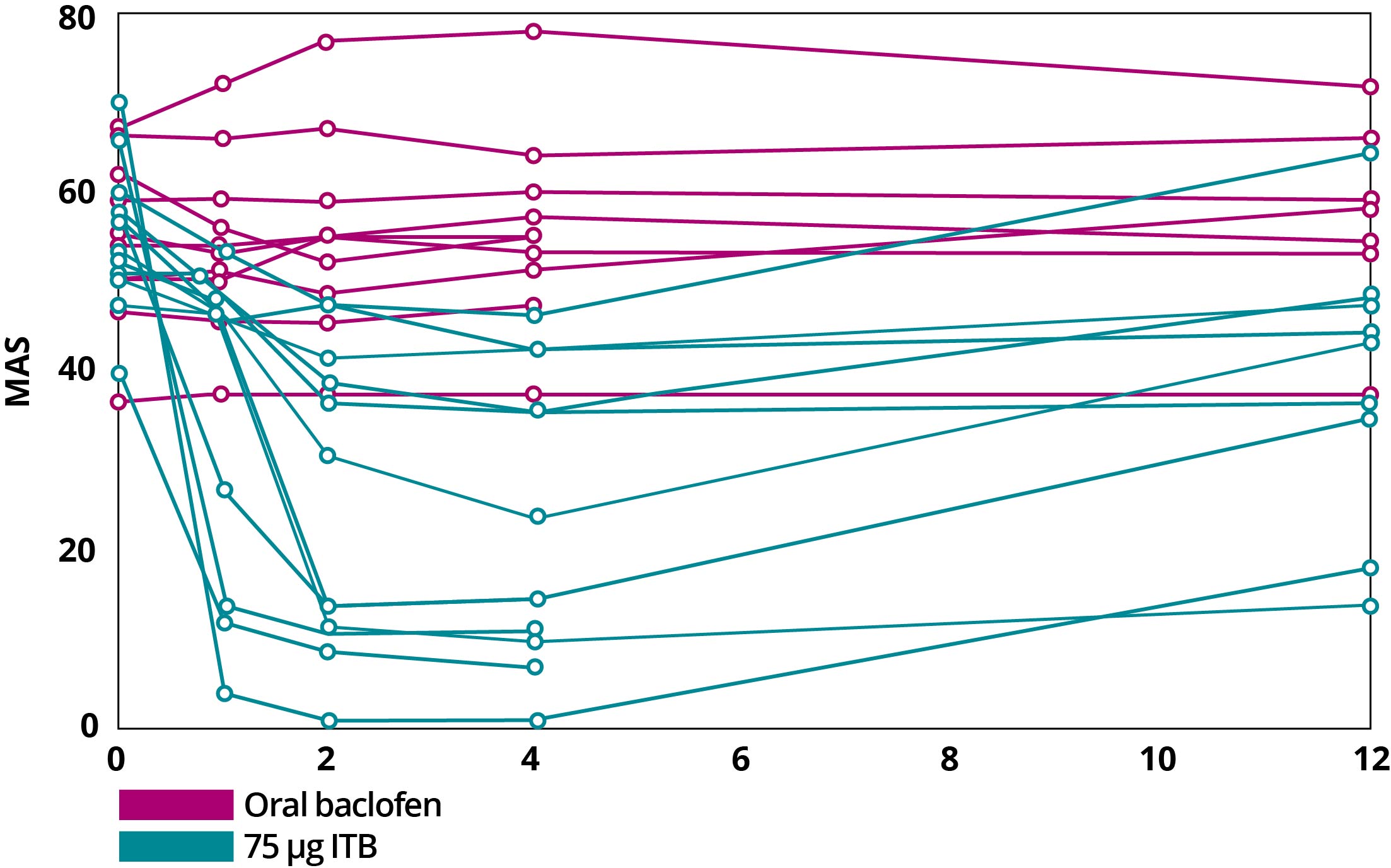
Adapted from Reference 4: Heetla HW, Proost JH, Molmans BH, Staal MJ, van Laar T. A pharmacokinetic-pharmacodynamic model for intrathecal baclofen in patients with severe spasticity. Br J Clin Pharmacol. 2016;81(1):101-112.
Frequency and Severity of Spasmsa in Patients With Spinal Cord Injury, Cerebral Palsy, and Stroke after 3 Years of Oral Baclofen or ITB Therapy4
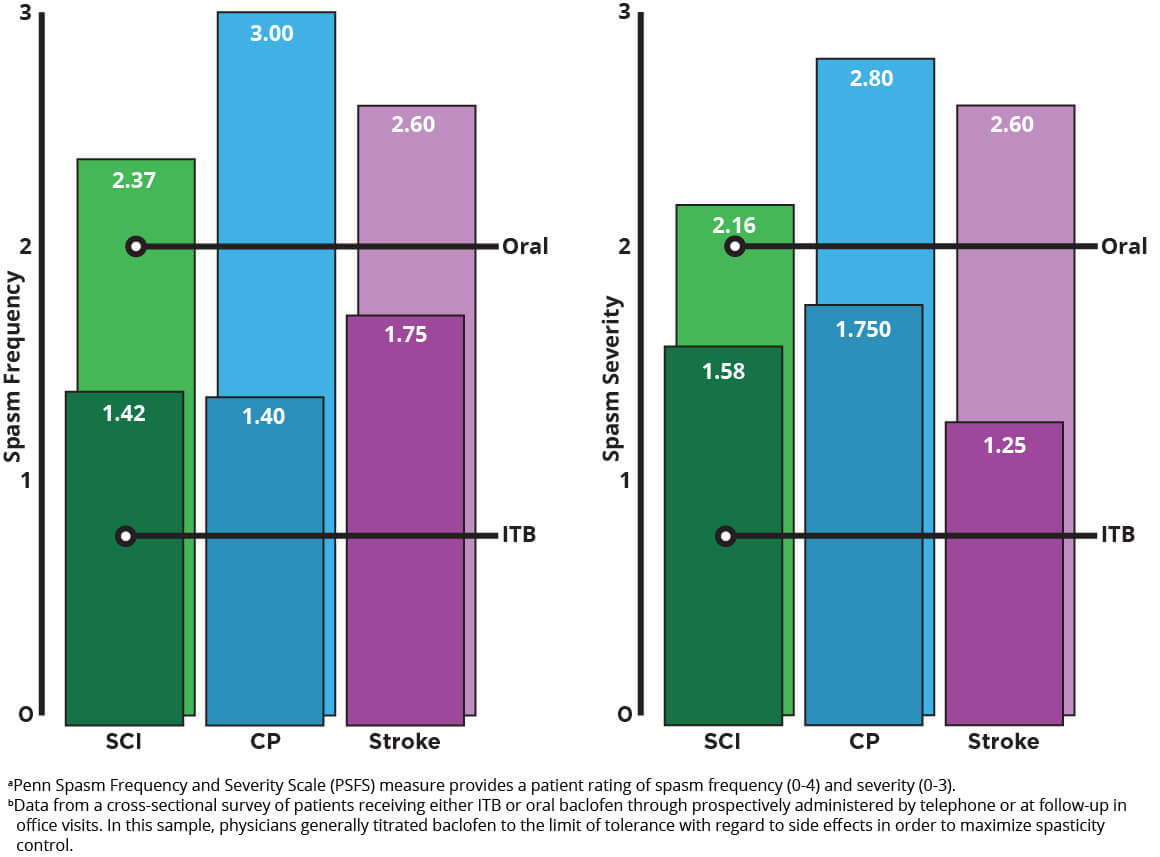
Adapted from Reference 4: Heetla HW, Proost JH, Molmans BH, Staal MJ, van Laar T. A pharmacokinetic-pharmacodynamic model for intrathecal baclofen in patients with severe spasticity. Br J Clin Pharmacol. 2016;81(1):101-112.
The most frequent drug adverse events vary by indication but include: hypotonia (34.7%), somnolence (20.9%), headache (10.7%), convulsion (10.0%), dizziness (8.0%), urinary retention (8.0%), nausea (7.3%), and paresthesia (6.7%). Dosing and programming errors may result in clinically significant overdose or withdrawal. Acute massive overdose may result in coma and may be life threatening.
Drowsiness has been reported in patients on Lioresal® Intrathecal. Patients should be cautioned regarding the operation of automobiles or other dangerous machinery and activities made hazardous by decreased alertness. Patients should also be cautioned that the central nervous system depressant effects of Lioresal® Intrathecal may be additive to those of alcohol and other CNS depressants.
- Lioresal® Intrathecal (baclofen injection) for intrathecal injection [prescribing information]. Saol Therapeutics, Roswell, Georgia; January 2019.
- KemstroTM (baclofen orally disintegrating tablets) for oral use [prescribing information]. Milwaukee, WI: Schwarz Pharma; Revised October 2003.
- McCormick ZL, Chu SK, Binler D, et al. Intrathecal versus oral baclofen: a matched cohort study of spasticity, pain, sleep, fatigue, and quality of life. PM R. 2016;8(6):553-562.
- Heetla HW, Proost JH, Molmans BH, Staal MJ, van Laar T. A pharmacokinetic-pharmacodynamic model for intrathecal baclofen in patients with severe spasticity. Br J Clin Pharmacol. 2016;81(1):101-112.