Withdrawal
When treating severe spasticity with Lioresal® Intrathecal (baclofen injection), it is mandatory that all patients, caregivers, and treating physicians receive adequate information regarding the risks of the mode of treatment. All patients receiving intrathecal baclofen therapy are potentially at risk for withdrawal. Except in overdose-related emergencies, the dose of Lioresal® Intrathecal should ordinarily be reduced slowly if the drug is discontinued for any reason.1
Prevention of abrupt discontinuation of intrathecal baclofen requires careful attention to programming and monitoring of the infusion system, refill scheduling and procedures, and pump alarms. Patients and caregivers should be advised of the importance of keeping scheduled refill visits and should be educated on the early symptoms of baclofen withdrawal.
What are the signs of withdrawal from Lioresal® Intrathecal?1
Early symptoms of baclofen withdrawal may include return of baseline spasticity, pruritus, hypotension, and paresthesias. Priapism may develop or recur if treatment with intrathecal baclofen is interrupted. Some clinical characteristics of the advanced intrathecal baclofen withdrawal syndrome may resemble autonomic dysreflexia, infection (sepsis), malignant hyperthermia, neuroleptic-malignant syndrome, or other conditions associated with a hypermetabolic state or widespread rhabdomyolysis.
Abrupt withdrawal of intrathecal baclofen, regardless of the cause, has resulted in sequelae that included high fever, altered mental status, exaggerated rebound spasticity, and muscle rigidity that in rare cases progressed to rhabdomyolysis, multiple organ-system failure, and death.
In the first 9 years of postmarketing experience, 27 cases of withdrawal temporally related to the cessation of baclofen therapy were reported; 6 patients died. In most cases, symptoms of withdrawal appeared within hours to a few days following interruption of baclofen therapy. Common reasons for abrupt interruption of intrathecal baclofen therapy included malfunction of the catheter (especially disconnection), low volume in the pump reservoir, and end of pump battery life; human error may have played a causal or contributing role in some cases.
How is withdrawal from Lioresal® Intrathecal managed?2
Rapid, accurate diagnosis and treatment in an emergency room or intensive-care setting are important to prevent the potentially life-threatening CNS and systemic effects of intrathecal baclofen withdrawal. The suggested treatment for intrathecal baclofen withdrawal is the restoration of intrathecal baclofen at or near the same dosage as before therapy was interrupted. However, if restoration of intrathecal delivery is delayed, treatment with GABA-ergic agonist drugs, such as oral or enteral baclofen, or oral, enteral, or intravenous benzodiazepines may prevent potentially fatal sequelae. Oral or enteral baclofen alone should not be relied upon to halt the progression of intrathecal baclofen withdrawal.
Seizures have been reported during overdose and with withdrawal from Lioresal® Intrathecal as well as in patients maintained on therapeutic doses of Lioresal® Intrathecal.
Evaluation for withdrawal can be performed using the presented algorithm. Symptoms of withdrawal can present within a few hours to 2 days and can vary not only in presentation but in degree of intensity (see Spectrum of Symptoms). Severity is not consistently related to dosing levels. The most common symptom is a return to a patient’s baseline hypertonia, but symptoms may be nonspecific and thus their presence may not be solely related to withdrawal. Due to the potential for multiorgan failure, patients with symptoms of withdrawal should be monitored closely.2
Spectrum of Withdrawal Symptoms2
MILD
• Increase in tone
• Pruritus without rash
• Irritability
MODERATE
• Return of pathologic tone
• Altered mental state
• Mild sysphoria
• Elevated creatine phosphokinase level
• Hypotension
• Paresthesia
• Fever
SEVERE
• Severe increase in tone
• Stupor and coma
• Rhabdomyolysis
Note that elevated or rising creatnine phosphokinase has been observed during withdrawal, but the sensitivity and specificity of this marker has not been formally assessed. Serum or CSF levels of baclofen are not considered a useful indicator of withdrawal.2
Definitive therapy for acute, severe withdrawal is restoration of intrathecal baclofen, and a panel of experts recommends this as first-line therapy. The dose administered and the frequency of dosing are dependent on patient-specific factors, including severity of withdrawal, previous dosing levels, time from onset of symptoms, and responses to prior bolus doses. If intrathecal exposure cannot be achieved promptly, oral medication should be considered. If catheter disruption is detected, surgical revision or replacement should be performed. Pump replacement or battery replacement should also be performed if malfunctions are detected.2
Emergent (Acute) Troubleshooting —Withdrawal2
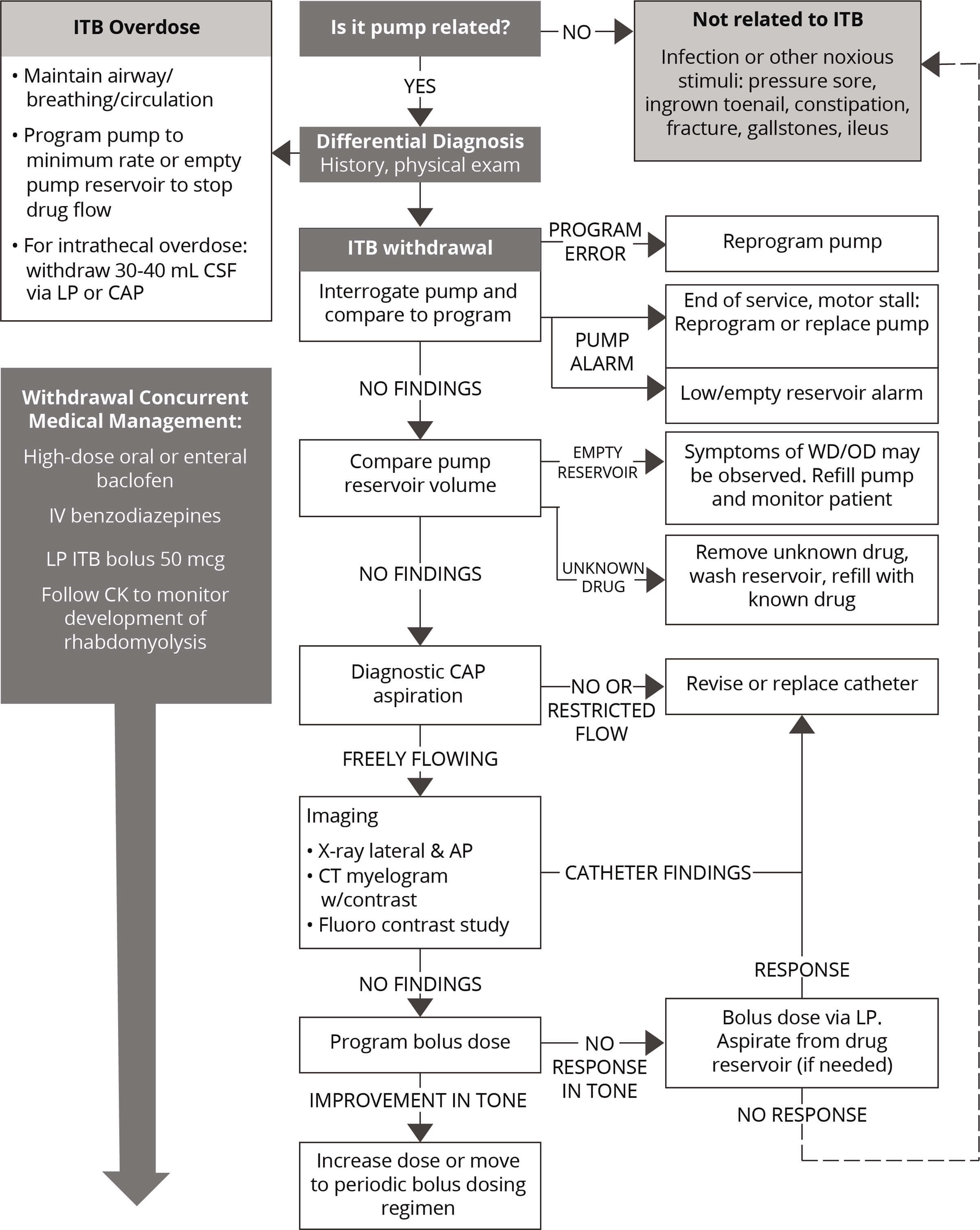
AP, anterior-posterior; CAP, catheter access port; CK, creatnine phosphokinase; CSF, cerebral spinal fluid; LP, lumbar puncture; WD/OD, withdrawal /overdose.
Adapted from Reference 2: Saulino M, Anderson DJ, Doble J, et al. Best practices for Intrathecal baclofen therapy: troubleshooting. Neuromodulation. 2016;19(6):632-641.
Early symptoms of baclofen withdrawal may include return of baseline spasticity, pruritus, hypotension and paresthesias. Priapism may develop or recur if treatment with intrathecal baclofen is interrupted. Signs of overdose may appear suddenly or insidiously, and a massive overdose may present as coma. Less sudden and/or less severe forms of overdose may present with signs of drowsiness, lightheadedness, dizziness, somnolence, respiratory depression, seizures, rostral progression of hypotonia and loss of consciousness progressing to coma.
Should overdose appear likely, the patient should be taken immediately to a hospital for assessment and emptying of pump reservoir. Except in overdose related emergencies, the dose of Lioresal® Intrathecal should ordinarily be reduced slowly if the drug is discontinued for any reason.
- Lioresal® Intrathecal (baclofen injection) for intrathecal injection [prescribing information]. Saol Therapeutics, Roswell, Georgia; January 2019.
- Saulino M, Anderson DJ, Doble J, et al. Best practices for intrathecal baclofen therapy: troubleshooting. Neuromodulation. 2016;19(6):632-641.