Dosing Initiation and Long-term Management-Recommended Practices
ITB Therapy℠ with Lioresal® Intrathecal may provide functional spasticity control in patients with severe spasticity that interferes with function, activity of daily living, comfort, positioning, or caregiving.1,2 Dosing options are flexible and should be tailored to meet individual patient needs in order to maintain tone as near normal as appropriate and as quickly and safely as possible.3
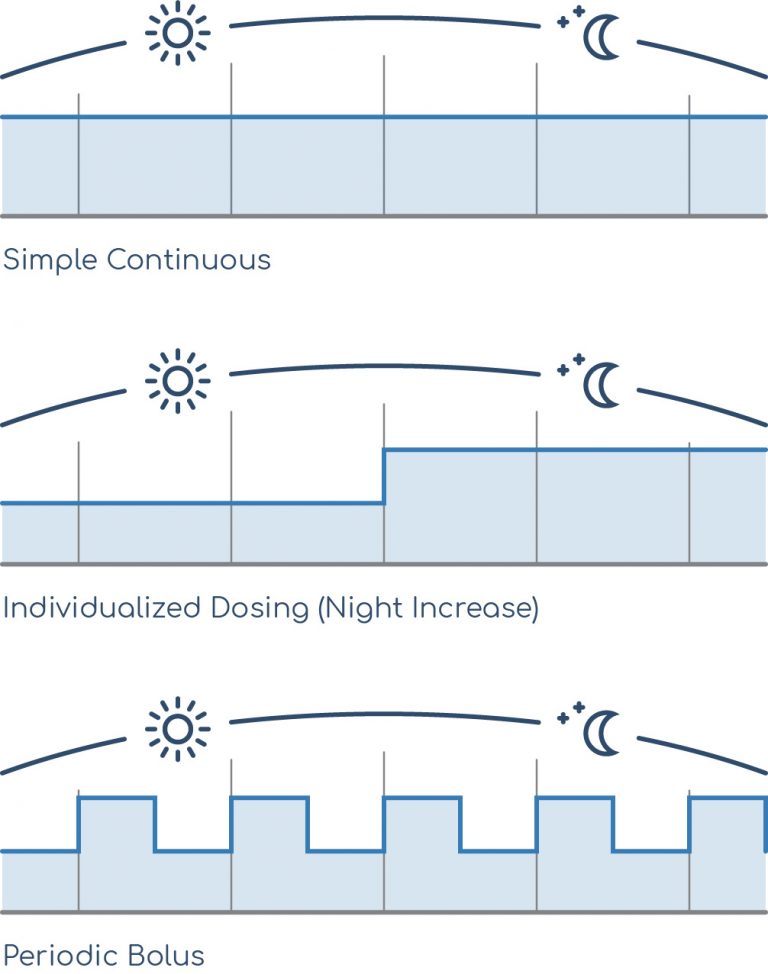
Sample Dosing With Programmable Pump

Lioresal® Intrathecal can be dosed through individualized, flexible dose modes with the programmable pump, including3,4
- Continuous (dose delivered continuously throughout a 24-hour period)
- Periodic bolus (regularly scheduled bolus doses within a 24-hour period)
Learn more about Dose Titration and Maintenance Therapy for Lioresal® Intrathecal.
Dose Titration
To individualize ITB Therapy℠ with Lioresal® Intrathecal, an initial total daily dose must be calculated and titrated appropriately.1 Drug delivery can begin as soon as the pump is implanted.4 Because therapy optimization requires multiple dose adjustments, it is recommended that the pump be filled
at time of surgery and a preimplant priming bolus be completed (for both new implants and replacement pumps).3 Patients must demonstrate a positive clinical response to a bolus dose of Lioresal® Intrathecal in a screening trial before proceeding to pump implantation and long-term use.1
Frequency and size of dose adjustments should be individualized, and patients and their caregivers should be educated about expectations during dose titration and long-term therapy, as well as the signs and symptoms of overdose or withdrawal.3,4
Lioresal® Intrathecal is most often administered in a continuous infusion mode via the programmable SynchroMed® II pump.1 Lioresal® Intrathecal is available in single-use ampules containing 50 mcg/mL, 500 mcg/mL, 2000 mcg/mL.1 Recent best practice publications recommend initiating therapy with the 500 mcg/mL concentration to provide maximum flexibility for dosing in the lower range without having to dilute the drug.3
The recommended titration schedule and amounts vary depending on the patient. Patients should be monitored closely in a fully equipped and fully staffed environment during the dose-titration period immediately following implant, and resuscitative equipment should be immediately available in case of life-threatening or intolerable side effects.1

The recommended initial total daily dose is double the screening dose that gave a positive effect administered over a 24-hour period unless the efficacy of the bolus screening dose was maintained for more than 8 hours. In these cases, the starting daily dose should be the screening dose delivered over a 24-hour period. No increased dose should be given in the first 24 hours.1
After the first 24 hours, the doses of Lioresal® Intrathecal may be titrated to achieve optimal dose based on assessment of the patient’s condition and progress toward individual goals.3
Titration Recommendations1
| Adult Patients | Pediatric Patients | |
Spasticity of Spinal Cord Origin |
Spasticity of Cerebral Origin |
|
Increase dose slowly by 10%-30% increments only once every 24 hours until desired clinical effect is achieved |
Increase dose slowly by 5%-15% increments only once every 24 hours until desired clinical effect is achieved |
Increase dose slowly by 5%-15% increments only once every 24 hours until desired clinical effect is achieved |
Tapering oral antispastic medications is recommended after the initiation of ITB Therapy℠ with Lioresal® Intrathecal. Patients can experience significant withdrawal during the titration period if they abruptly discontinue these medications. Reduction and discontinuation of oral antispasmotics should be done slowly and with careful monitoring by the physician. Abrupt reduction or discontinuation of concomitant antispastics should be avoided. Oral baclofen should remain available during ITB therapy dose titration and after the establishment of a stable dose in case management of ITB therapy withdrawal is needed.1,3
Titration may be considered complete when the patient is weaned from oral antispasmodics, the patient has achieved an optimal dose of Lioresal® Intrathecal, and/or additional dose increases do not provide further benefit or result in unwanted side effects. Stable therapeutic dose varies widely depending on the diagnosis, level of function, and goals of each individual.3
Long-term Maintenance Therapy
The clinical goal of ITB therapy is to maintain muscle tone as close to normal as possible, minimizing the frequency and severity of spasms to the extent possible without inducing intolerable side effects. When an optimal dose is achieved, it is important to monitor patients to see if they are meeting these goals. Oftentimes the maintenance dose needs to be adjusted during the first few months of therapy while patients adjust to changes in lifestyle due to the alleviation of spasticity.1
Periodically, the pump reservoir needs to be refilled. During this time, the daily dose may be increased to maintain adequate symptom control. The recommended amount of increase varies depending on the origin of spasticity. In addition, patients may require gradual increases in dose over time to maintain optimal response during chronic therapy. A sudden need for dose escalation suggests a catheter complication (eg, kink or dislodgement). Optimization of dose requires individual titration; the lowest dose with optimal response should be used.1
Patients and caregivers should be advised of the importance of keeping scheduled refill visits and should be educated on the early symptoms of baclofen withdrawal.
Patients should be infection-free prior to both a screening trial and a pump implantation. The presence of infection may interfere with an assessment of the patient’s response to bolus Lioresal® Intrathecal, increase the risk of surgical complications and complicate dosing.
Maintenance Therapy Dosing Recommendations1
| Adult Patients | Pediatric Patients | |
| Spasticity of Spinal Cord Origin | Spasticity of Cerebral Origin | |
|
|
|
- Lioresal® Intrathecal (baclofen injection) for intrathecal injection [prescribing information]. Saol Therapeutics, Roswell, Georgia; January 2019.
- Saulino M, Ivanhoe CB, McGuire JR, et al. Best practices for intrathecal baclofen therapy: patient selection. Neuromodulation. 2016;19(6):607-615.
- Boster AL, Adair RL, Gooch JL, et al. Best practices for intrathecal baclofen therapy: dosing and long-term management. Neuromodulation. 2016;19(6):623-631.
- Francisco GE, Saulino M. Chapter 19: Intrathecal baclofen for spasticity. In: Brashear A, Elovic E, eds. Spasticity: Diagnosis and Management. 2nd ed. New York, NY: Demos Medical Publishing, LLC, 2016.