Efficacy
ITB Therapy℠ with Lioresal® Intrathecal (baclofen injection) has a well-established efficacy profile for severe spasticity. It is approved to treat severe spasticity of both cerebral and spinal origins.1
Before implantation of the SynchroMed® II infusion system, patients must show a positive response to Lioresal® Intrathecal in a screening trial.

Efficacy for Severe Spasticity of Spinal Origin

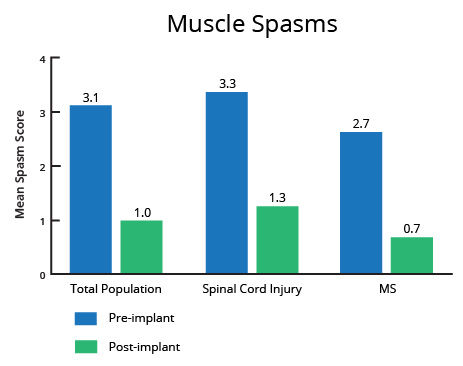
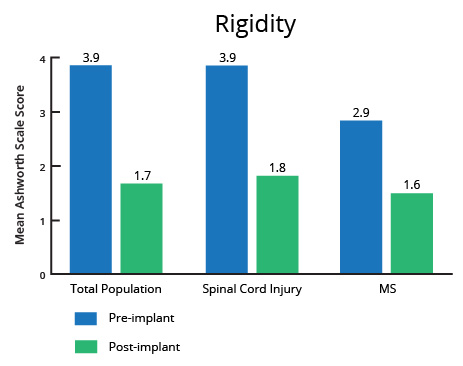
A controlled clinical investigation (Penn et al. 1989) in patients with severe spasticity due to multiple sclerosis (MS) and spinal cord injury (SCI) demonstrated the efficacy and safety of ITB Therapy℠ with Lioresal® Intrathecal and served as the basis for US Food and Drug Administration (FDA) approval for severe spasticity of spinal origin spasticity in 1992. This study compared the effects of either a single intrathecal dose or a 3-day infusion of Lioresal® Intrathecal to placebo, demonstrating the superiority of Lioresal® Intrathecal in change from baseline in the Ashworth Scale for spasticity and the change from baseline in the frequency of spasms.1
This study was supported by additional studies, including studies in patients with spasticity from cerebral origin, pediatric patients, screening trials, and open-label, long-term (up to 7 years) extension trials (see Penn 1992; Coffey et al. 1993).
Efficacy for Severe Spasticity of Cerebral Origin
The efficacy of Lioresal® Intrathecal was investigated in 3 controlled clinical trials: 2 trials enrolled patients with cerebral palsy and 1 enrolled patients with spasticity due to previous brain injury or stroke. These trials were the basis of FDA approval for spasticity of cerebral origin in 1996, as they demonstrated significant reduction in spasticity. Note that there is no safety or efficacy data in children under 4 years of age or in patients less than 1 year after traumatic brain injury.1
The first study, a randomized controlled crossover trial of 51 patients with cerebral palsy, and demonstrated statistically significant superiority of Lioresal® Intrathecal compared to placebo in reducing spasticity as measured by the Ashworth Scale (see Gilmartin et al. 2000). This study was conducted after Albright et al. published data demonstrating efficacy of ITB Therapy℠ with Lioresal® Intrathecal in both pediatric and adult patients with cerebral palsy.
A second crossover study was conducted in 11 patients with spasticity arising from brain injury. Despite the small sample size, the study yielded a nearly significant test statistic (P=0.066) and provided directionally favorable results (see Meythaler et al. 1996). The last study, however, did not provide data that could be reliably analyzed.1
Supportive data and long-term efficacy of ITB Therapy℠ with Lioresal® Intrathecal were demonstrated in additional trials.

The most frequent drug adverse events vary by indication but include: hypotonia (34.7%), somnolence (20.9%), headache (10.7%), convulsion (10.0%), dizziness (8.0%), urinary retention (8.0%), nausea (7.3%), and paresthesia (6.7%). Dosing and programming errors may result in clinically significant overdose or withdrawal. Acute massive overdose may result in coma and may be life threatening. Drowsiness has been reported in patients on Lioresal® Intrathecal. Patients should be cautioned regarding the operation of automobiles or other dangerous machinery and activities made hazardous by decreased alertness. Patients should also be cautioned that the central nervous system depressant effects of Lioresal® Intrathecal may be additive to those of alcohol and other CNS depressants. Seizures have been reported during overdose and with withdrawal from Lioresal® Intrathecal as well as in patients maintained on therapeutic doses of Lioresal® Intrathecal. Fatalities have been reported with Lioresal® Intrathecal use. The most frequent and serious adverse events related to device and implant procedures are catheter dislodgement from the intrathecal space, catheter break/cut, and implant site infection including meningitis. Electromagnetic interference (EMI) and magnetic resonance imaging (MRI) may cause patient injury, system damage, operational changes to the pump, and changes in flow rate.
- Lioresal® Intrathecal (baclofen injection) for intrathecal injection [prescribing information]. Saol Therapeutics, Roswell, Georgia; January 2019.
- Penn RD, Savoy SM, Corcos D, et al. Intrathecal baclofen for severe spinal spasticity. N Engl J Med. 1989;320(23):1517-1521.
- Coffey RJ, Cahill D, Steers W, et al. Intrathecal baclofen for intractable spasticity of spinal origin: results of a long-term multicenter study. J Neurosurg. 1993;78(2):226-232.
- Penn RD. Intrathecal baclofen for spasticity of spinal origin: seven years of experience. J Neurosurg. 1992;77(2):236-240.
- Azouvi P, Mane M, Thiebaut JB, Denys P, Remy-Neris O, Bussel B. Intrathecal baclofen administration for control of severe spinal spasticity: functional improvement and long-term follow-up. Arch Phys Med Rehabil. 1996;77(1):35-39.
- Dario A, Scamoni C, Bono G, Ghezzi A, Zaffaroni M. Functional improvement in patients with severe spinal spasticity treated with chronic intrathecal baclofen infusion. Funct Neurol. 2001;16(4):311-315.
- Draulans N, Vermeersch K, Degraeuwe B, et al. Intrathecal baclofen in multiple sclerosis and spinal cord injury: complications and long-term dosage evolution. Clin Rehabil. 2013;27(12):1137-1143.
- Gianino JM, York MM, Paice JA, Shott S. Quality of life: effect of reduced spasticity from intrathecal baclofen. J Neurosci Nurs. 1998;30(1):47-54.
- Guglielmino A, Sorbello M, Fazzio S, et al. Continuous intrathecal baclofen administration by a fully implantable electronic pump for severe spasticity treatment: our experience. Minerva Anestesiol. 2006;72(10):807-820.
- Khan AA, Birks-Agnew I, Bullock P, Rushton D. Clinical outcome and complications of intrathecal baclofen pump in multiple sclerosis patients: a retrospective study. NeuroRehabilitation. 2010;27(2):117-120.
- Loubser PG, Narayan RK, Sandin KJ, Donovan WH, Russell KD. Continuous infusion of intrathecal baclofen: long-term effects on spasticity in spinal cord injury. Paraplegia. 1991;29(1):48-64.
- Middel B, Kuipers-Upmeijer H, Bouma J, et al. Effect of intrathecal baclofen delivered by an implanted programmable pump on health related quality of life in patients with severe spasticity. J Neurol Neurosurg Psychiatry. 1997;63(2):204-209.
- Ordia JI, Fischer E, Adamski E, Spatz EL. Chronic intrathecal delivery of baclofen by a programmable pump for the treatment of severe spasticity. J Neurosurg. 1996;85(3):452-457.
- Ordia JI, Fischer E, Adamski E, Chagnon KG, Spatz EL. Continuous intrathecal baclofen infusion by a programmable pump in 131 consecutive patients with severe spasticity of spinal origin. Neuromodulation. 2002;5(1):16-24.
- Vender JR, Hughes M, Hughes BD, Hester S, Holsenback S, Rosson B. Intrathecal baclofen therapy and multiple sclerosis: outcomes and patient satisfaction. Neurosurg Focus. 2006;21(2):e6.
- Vidal J, Fenollosa P, Martin E, et al. Safety and efficacy of intrathecal baclofen infusion by implantable pump for the treatment of severe spinal spasticity: a spanish multicenter study. Neuromodulation. 2000;3(4):175-182.
- Zahavi A, Geertzen JH, Middel B, Staal M, Rietman JS. Long term effect (more than five years) of intrathecal baclofen on impairment, disability, and quality of life in patients with severe spasticity of spinal origin. J Neurol Neurosurg Psychiatry. 2004;75(11):1553-1557.
- Gilmartin R, Bruce D, Storrs BB, et al. Intrathecal baclofen for management of spastic cerebral palsy: multicenter trial. J Child Neurol. 2000;15(2):71-77.
- Albright AL, Cervi A, Singletary J. Intrathecal baclofen for spasticity in cerebral palsy. JAMA. 1991;265(11):1418-1422.
- Meythaler JM, DeVivo MJ, Hadley M. Prospective study on the use of bolus intrathecal baclofen for spastic hypertonia due to acquired brain injury. Arch Phys Med Rehabil. 1996;77(5):461-466.
- Albright AL, Gilmartin R, Swift D, Krach LE, Ivanhoe CB, McLaughlin JF. Long-term intrathecal baclofen therapy for severe spasticity of cerebral origin. J Neurosurg. 2003;98(2):291-295.
- Becker R, Alberti O, Bauer BL. Continuous intrathecal baclofen infusion in severe spasticity after traumatic or hypoxic brain injury. J Neurol. 1997;244(3):160-166.
- Borowski A, Shah SA, Littleton AG, Dabney KW, Miller F. Baclofen pump implantation and spinal fusion in children: techniques and complications. Spine (Phila Pa 1976). 2008;33(18):1995-2000.
- Borowski A, Littleton AG, Borkhuu B, et al. Complications of intrathecal baclofen pump therapy in pediatric patients. J Pediatr Orthop. 2010;30(1):76-81.
- Dario A, Di Stefano MG, Grossi A, Casagrande F, Bono G. Long-term intrathecal Baclofen infusion in supraspinal spasticity of adulthood. Acta Neurol Scand. 2002;105(2):83-87.
- Francisco GE, Boake C. Improvement in walking speed in poststroke spastic hemiplegia after intrathecal baclofen therapy: a preliminary study. Arch Phys Med Rehabil. 2003;84(8):1194-1199.
- Ghosh D, Mainali G, Khera J, Luciano M. Complications of intrathecal baclofen pumps in children: experience from a tertiary care center. Pediatr Neurosurg. 2013;49(3):138-144.
- Hoving MA, van Raak EP, Spincemaille GH, et al. Efficacy of intrathecal baclofen therapy in children with intractable spastic cerebral palsy: a randomised controlled trial. Eur J Paediatr Neurol. 2009;13(3):240-246.
- Hoving MA, van Raak EP, Spincemaille GH, et al. Safety and one-year efficacy of intrathecal baclofen therapy in children with intractable spastic cerebral palsy. Eur J Paediatr Neurol. 2009;13(3):247-256.
- Ivanhoe CB, Francisco GE, McGuire JR, Subramanian T, Grissom SP. Intrathecal baclofen management of poststroke spastic hypertonia: implications for function and quality of life. Arch Phys Med Rehabil. 2006;87(11):1509-1515.
- Krach LE, Nettleton A, Klempka B. Satisfaction of individuals treated long-term with continuous infusion of intrathecal baclofen by implanted programmable pump. Pediatr Rehabil. 2006;9(3):210-218.
- Meythaler JM, McCary A, Hadley MN. Prospective assessment of continuous intrathecal infusion of baclofen for spasticity caused by acquired brain injury: a preliminary report. J Neurosurg. 1997;87(3):415-419.
- Meythaler JM, Guin-Renfroe S, Grabb P, Hadley MN. Long-term continuously infused intrathecal baclofen for spastic-dystonic hypertonia in traumatic brain injury: 1-year experience. Arch Phys Med Rehabil. 1999;80(1):13-19.
- Meythaler JM, Guin-Renfroe S, Law C, Grabb P, Hadley MN. Continuously infused intrathecal baclofen over 12 months for spastic hypertonia in adolescents and adults with cerebral palsy. Arch Phys Med Rehabil. 2001;82(2):155-161.
- Morton RE, Gray N, Vloeberghs M. Controlled study of the effects of continuous intrathecal baclofen infusion in non-ambulant children with cerebral palsy. Dev Med Child Neurol. 2011;53(8):736-741.
- Motta F, Buonaguro V, Stignani C. The use of intrathecal baclofen pump implants in children and adolescents: safety and complications in 200 consecutive cases. J Neurosurg. 2007;107(1 Suppl):32-35.
- Motta F, Antonello CE, Stignani C. Intrathecal baclofen and motor function in cerebral palsy. Dev Med Child Neurol. 2011;53(5):443-448.
- Ordia JI, Fischer E, Adamski E, Spatz EL. Continuous intrathecal baclofen infusion delivered by a programmable pump for the treatment of severe spasticity following traumatic brain injury. Neuromodulation. 2002;5(2):103-107.
- Ramstad K, Jahnsen R, Lofterod B, Skjeldal OH. Continuous intrathecal baclofen therapy in children with cerebral palsy - when does improvement emerge? Acta Paediatr. 2010;99(11):1661-1665.
- Schiess MC, Oh IJ, Stimming EF, et al. Prospective 12-month study of intrathecal baclofen therapy for poststroke spastic upper and lower extremity motor control and functional improvement. Neuromodulation. 2011;14(1):38-45; discussion 45.
- Shilt JS, Reeves S, Lai LP, et al. The outcome of intrathecal baclofen treatment on spastic diplegia: Preliminary results with a minimum of two year follow-up. J Pediatr Rehabil Med. 2008;1(3):255-261.
- Vles GF, Soudant DL, Hoving MA, et al. Long-term follow-up on continuous intrathecal Baclofen therapy in non-ambulant children with intractable spastic Cerebral Palsy. Eur J Paediatr Neurol. 2013;17(6):639-644.