Troubleshooting
ITB Therapy℠ with Lioresal® Intrathecal has a well-established safety and efficacy profile in the treatment of severe spasticity demonstrated in the 20+ years of clinical use.1 However, patients should be monitored and periodically reevaluated to ensure optimal therapy.
In general, patients with chronic, nonprogressive neurologic conditions, dosing of Lioresal® Intrathecal (baclofen injection) should be relatively stable during the maintenance phase of therapy. More frequent dose evaluations and
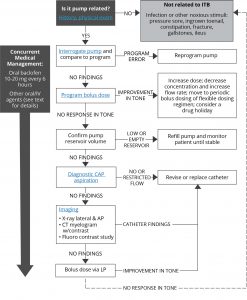
adjustments may be necessary in patients with progressive diseases (eg, multiple sclerosis). When patients present with previously well-controlled hypertonia or when they present with hypotonia, their spasticity should be reevaluated and their ITB therapy system should be reexamined. If no patient-related factors for change in spasticity are discovered, an investigation into their ITB therapy system should be undertaken. An algorithm for loss of efficacy has recently been developed. Learn more by clicking on each box in the image.2,3
Loss of Efficacy Troubleshooting Algorithm3
AP, anterior-posterior; CAP, catheter access port; CT, computed tomography; ITB, intrathecal; IV, intravenous; LP, lumbar puncture.
Adapted from Reference 3: Saulino M, Anderson DJ, Doble J, et al. Best practices for Intrathecal baclofen therapy: troubleshooting. Neuromodulation. 2016;19(6):632-641
Reservoir refilling must be performed by fully trained and qualified personnel following the directions provided by the pump manufacturer. Extreme caution must be used when filling an FDA approved implantable pump, following strict aseptic technique and ensuring refill directly into the reservoir and not the catheter access port. Following pump implantation, and for each adjustment of the dosing rate of the pump and/or concentration of Lioresal® Intrathecal, the patient should be monitored closely until it is certain the patient’s response to the infusion is acceptable and reasonably stable.
- Boster AL, Bennett SE, Bilsky GS, et al. Best practices for intrathecal baclofen therapy: screening test. Neuromodulation. 2016;19(6):616-622.
- Francisco GE, Saulino M. Chapter 19: Intrathecal baclofen for spasticity. In: Brashear A, Elovic E, eds. Spasticity: Diagnosis and Management. 2nd ed. New York, NY: Demos Medical Publishing, LLC, 2016.
- Saulino M, Anderson DJ, Doble J, et al. Best practices for intrathecal baclofen therapy: troubleshooting. Neuromodulation. 2016;19(6):632-641.
- Lioresal® Intrathecal (baclofen injection) for intrathecal injection [prescribing information]. Saol Therapeutics, Roswell, Georgia; January 2019.