Consider a Screening Trial
Many patients living with severe spasticity experience unacceptable side effects, such as drowsiness, or inconsistent efficacy in managing their severe spasticity when taking oral baclofen.1 When oral baclofen, or other treatments, do not provide the desired results, ITB Therapy℠ with Lioresal® Intrathecal (baclofen injection) offers another treatment option.
With more than 3 decades of use, ITB Therapy℠ with Lioresal® Intrathecal is well documented as an effective and reliable treatment of severe spasticity.2 Lioresal® Intrathecal offers the unique opportunity to try out intrathecal baclofen to see how it affects severe spasticity in a 1-day screening trial.
See if targeted drug delivery with Lioresal® Intrathecal could benefit your patients with severe spasticity by participating in a 1-day screening trial.
| Who is a candidate for a screening trial of Lioresal® Intrathecal?1,3 |
|
|
|
|
|
|
| A screening trial with Lioresal® Intrathecal is an important step that can allow patients to2 |
|
|
|
|
Functional improvement during the 1-day screening trial may vary depending on the person.2 It is even possible that some patients may lose functional ability temporarily during the screening trial due to underlying muscle weakness that becomes apparent when spasticity is reduced. This can be a normal response, and expectations of further treatment with Lioresal® Intrathecal may include a rehabilitation plan to strengthen weak muscles and improve function.
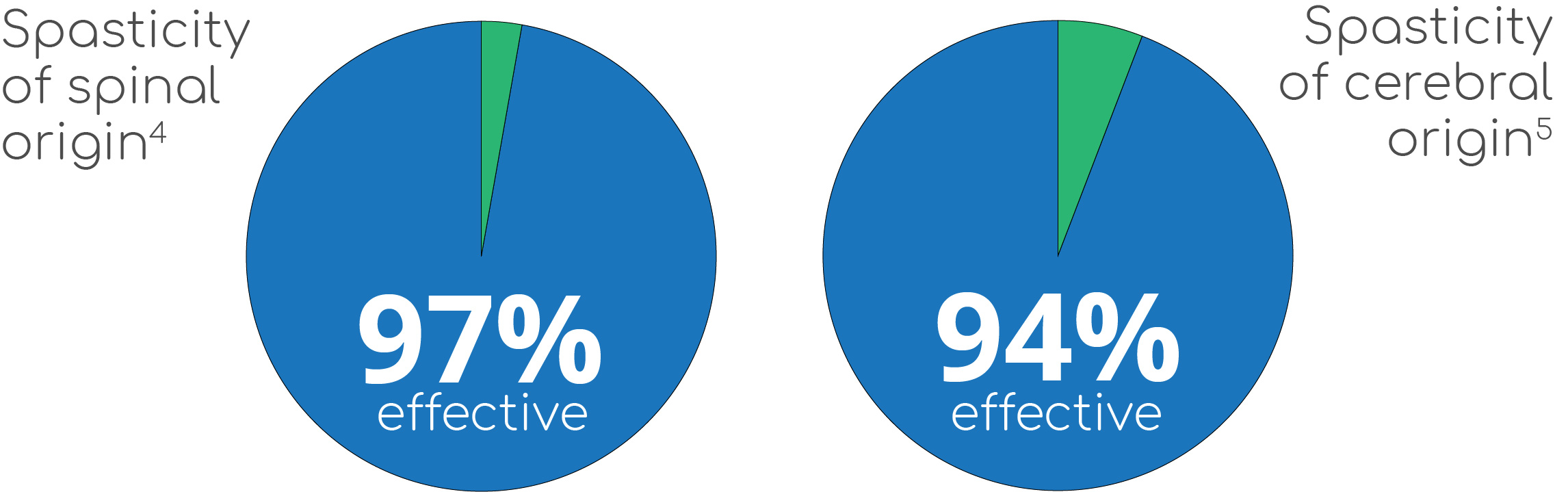
The one-day Lioresal® Intrathecal screening trial offers important information that could lead to improved care for many patients who live with severe spasticity. The screening dose has been shown to be effective in 97% of patients with spasticity of spinal origin4 and in 94% of patients with spasticity of cerebral origin.5
Keep in mind that this is not a treatment in itself; it is a test to see how further treatment with Lioresal® Intrathecal can affect spasticity.2 Good candidates for a treatment with Lioresal® Intrathecal are those who are clinically stable, understand the risks and benefits associated with ITB Therapy℠ with Lioresal® Intrathecal, are able to return to the clinic for dosage titration and periodic pump refills, have demonstrated a positive response in the screening trial.3,6
Lioresal® Intrathecal is intended for use by the intrathecal route in single bolus test doses (via spinal catheter or lumbar puncture) and, for chronic use, only in implantable pumps approved by the FDA specifically for the administration of Lioresal® Intrathecal into the intrathecal space. Lioresal® Intrathecal is not recommended for intravenous, intramuscular, subcutaneous or epidural administration. An attempt should be made to discontinue concomitant oral antispasticity medication to avoid possible overdose or adverse drug interactions, either prior to screening or following implant and initiation of chronic Lioresal® Intrathecal infusion. Following pump implantation, and for each adjustment of the dosing rate of the pump and/or concentration of Lioresal® Intrathecal, the patient should be monitored closely until it is certain the patient’s response to the infusion is acceptable and reasonably stable.
- Saulino M, Ivanhoe CB, McGuire JR, et al. Best practices for intrathecal baclofen therapy: patient selection. Neuromodulation. 2016;19(6):607-615.
- Boster AL, Bennett SE, Bilsky GS, et al. Best practices for intrathecal baclofen therapy: screening test. Neuromodulation. 2016;19(6):616-622.
- Francisco GE, Saulino M. Chapter 19: Intrathecal baclofen for spasticity. In: Brashear A, Elovic E, eds. Spasticity: Diagnosis and Management. 2nd ed. New York, NY: Demos Medical Publishing, LLC, 2016.
- Penn R. Intrathecal baclofen for spasticity of spinal origin: seven years of experience. J Neurosurg. 1992;77:236-240.
- Gilmartin R, Bruce D, Storrs B, et al. Intrathecal baclofen for management of spastic cerebral palsy: Multicenter trial. J Child Neurol. 2000;15(2):71-77.
- Lioresal® Intrathecal (baclofen injection) for intrathecal injection [prescribing information]. Saol Therapeutics, Roswell, Georgia; January 2019.